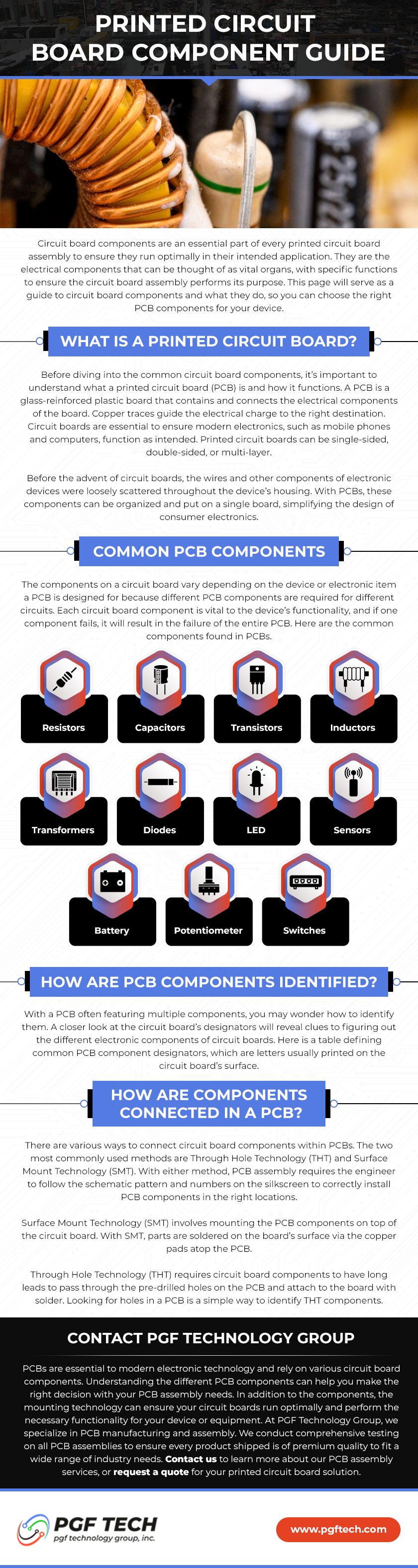
What are the Biggest ISO 13485:2016 Benefits?
ISO 13485:2016 is an international standard for quality management systems specific to the medical device industry. It is based on the generic ISO 9001:2015 standard, but with additional requirements that are specific to the medical device industry.
The standard is particularly relevant for companies that manufacture electronic assemblies for use in medical devices, as these assemblies must meet strict regulatory requirements and high-quality standards to ensure the safety and efficacy of the final product.
Meet Requirements
One of the main benefits of ISO 13485:2016 for electronic assembly manufacturers is that it helps them to meet regulatory requirements. Medical devices are subject to strict regulations, both in terms of product design and manufacturing. ISO 13485:2016 provides a framework for organizations to demonstrate that they are meeting these requirements, which can make it easier to obtain regulatory approvals and market their products.
Improve Quality
Another benefit of ISO 13485:2016 for electronic assembly manufacturers is that it helps them to improve their overall product quality. The standard requires organizations to establish and maintain processes for controlling the design, development, production, and delivery of their products. This ensures that products are high quality and meet the requirements of customers and other stakeholders.
Improve the Risk Management Process
ISO 13485:2016 also helps electronic assembly manufacturers to improve their risk management processes. The standard requires organizations to identify and evaluate risks associated with their products, and to implement measures to control these risks. This can help to reduce the likelihood of product defects and recalls, which can be costly and damaging to a company’s reputation.
Additionally, for electronic assembly manufacturers, the standard requires ensuring that the electronic assemblies are in accordance with safety standards such as IEC 60601-1 and EMC standards. This guarantees that the assemblies conform to the requirements of the medical device, thus ensuring the safety and efficacy of the final product.
Certified Safe Medical Devices
For medical device customers, one of the main benefits of working with electronic assembly manufacturers that are certified to ISO 13485:2016 is that they can be confident that the products they receive are of high quality and meet regulatory requirements. This can help to reduce the risk of product defects and recalls, and ensure that the electronic assemblies are safe and effective for use in medical devices.
Conclusion
In conclusion, ISO 13485:2016 provides a framework for electronic assembly manufacturers to demonstrate that they meet regulatory requirements, improve product quality, manage risks effectively, ensure compliance with safety and EMC standards, improve internal processes, and ultimately improve their overall performance. Medical device customers who work with manufacturers that are certified to ISO 13485:2016 can be confident that the electronic assemblies they receive are safe, effective, and meet industry standards.
It is important to use an experienced printed circuit board assembly (PCBA) manufacturer like PGF Technology Group to ensure that your PCBs are made to the highest quality standards and meet your specific requirements. With years of experience and state-of-the-art machinery, PGF Technology Group is well-equipped to handle all your PCBA needs. They have a team of experts who are knowledgeable in all aspects of the PCB assembly process.