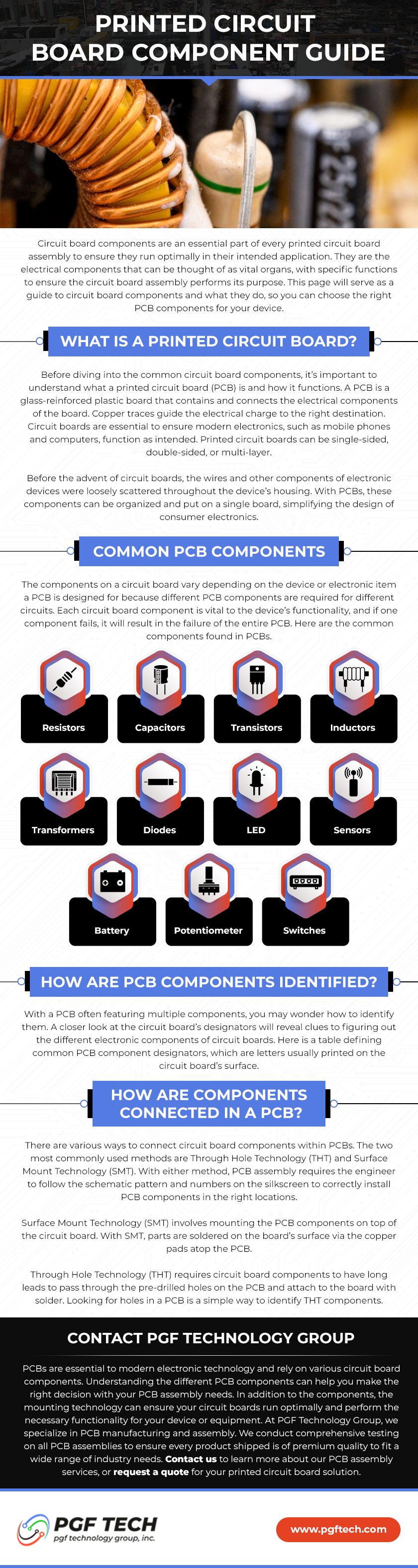
What is ISO 13485?
ISO 13485 is a medical device manufacturing certification that ensures customers of the regulations and procedures necessary for product development. This certification applies to the medical industry manufacturing of wire and cable connectors, surface mount and through-hole printed circuit boards, cable, wire processing, wire harness, and panel wire assemblies. Accordingly, issued on October 29, 2021, PGF Technology is an ISO-certified company that provides many benefits for all medical industry customers.
What are the Medical Certification Benefits?
Manufacturing medical devices and similar services require immense precaution and regulatory action to provide customers and patients with the most efficient products. With a variety of potential processes and safety measures, medical certifications allow quality management systems to follow established appropriate measures. As a medical assembly certification, ISO 13485 benefits manufacturing plants and customers in providing transparent safety regulations. After all, customers seeking manufacturers to complete various medical projects are more inclined to work with ISO-certified companies as they are guaranteed proper processing and effective medical devices.
Worldwide Access
The need for medical products, services, and industry manufacturers is a global and continuous market. To gain customers from all around the world, companies must provide effective products assembled from efficient processes. The medical certification provides customers the relief of knowing the necessary manufacturing assembly tactics are in place. Similarly, manufacturers gain more access to worldwide markets. With a standard certification, companies meet quality requirements to advertise their services internationally. Customer acquisition is more easily attainable, while potential customers increase with the ability of global outreach.
Process Improvement
As the manufacturing processes are standardized, companies continue improving efficiency and safety. Additionally, other services offered by the company can follow a similar assembly protocol showcasing improvement across the organization.
Increased Efficiency
Standard certification regulations help to increase efficiency within the companies while providing the ability to monitor supply chain performance. Manufacturers have clear process guidelines to follow, allowing for faster and more productive assemblies.
Safety
The ISO certification demonstrates safer and more effective medical devices due to the safety measures in place. The medical industry requires immense precautions to ensure the success of all delivered products. Additionally, manufacturers provide transparent and credible processes for each project and customer concerning medical equipment.
Regulation Requirements
To receive the ISO 13485 certification, companies must meet the regulatory requirements, as well as essentially the customer expectations. Arguably the most critical aspect of the medical certifications, the medical service requirements provides the credibility of manufacturers. All in all, the medical industry globally focuses on the safety and requirements of assembly to claim a successful manufacturing process.
PGF Medical Service Expertise
As can be seen, PGF Technology’s ISO certification demonstrates the company’s devotion to credibility, transparency, and quality products and services. Effectively, customers’ satisfaction and product functionality is the highest priority during all medical projects. To learn more about the printed circuit board medical assemblies and prototype services, PGF provides free downloadable eBooks.